Update: Novel spinal deformities in Norway
In response to a complaint regarding an advertorial titled, “Vaccine Breakthrough” that Elanco placed in Fish Farmer Yearbook 2021, we wish to clarify that all the data and conclusions written in that advertorial are founded solely on experience in Norwegian salmon operations.
One of the main references used for the advertorial was a review commissioned by the Norwegian Ministry of Trade, Industry and Fisheries from the Norwegian Veterinary Institute. The objective of the review was to verify one of the allegations made by various Norwegian farmers against compulsory PD vaccination in light of the side effects, in the form of cross stitch spinal deformities, they had experienced following the use of two oil-adjuvanted PD vaccines. The certified translation of the letter summarising the review is hereby presented in full.
Introduction
In a letter dated 27 May 2020 from the Norwegian Ministry of Trade, Industry and Fisheries (NFD) to the Norwegian Veterinary Institute (VI), the NFD writes the following:
“With regard to instructions concerning the vaccination of fish against PD, it has emerged that the incidence of adverse events may be higher than previously thought. This has caused the Ministry to request a technical assessment from the Norwegian Veterinary Institute.
We make reference to the discussions we had with the Veterinary Institute regarding an introductory study to determine the incidence of adverse events and impacts on fish welfare (dive reference 19112399). We have attached to Veterinary Institute’s project outline”
Reference is made to a project outline for the analysis of data from the aquaculture industry and vaccine companies dated 15 May 2020 from the Veterinary Institute. On the basis of one such analysis, the NFD asks the VI to assess how the vaccination order (the PD regulation, Section 7) will affect the welfare of vaccinated fish. The NFD also writes that the assessment should include results from other relevant research projects, including an ongoing project led by NOFIMA.
A project group at the VI was established for the assignment on 24 June and the group has carried out a survey of existing information/data. All fish placed in the sea in Norway are vaccinated against the most common bacterial diseases. Most available PD vaccines are 1-component and therefore a different vaccine (typically 6-component) will always additionally be used. As of now, the following PD vaccines are available on the Norwegian market:
MSD Animal Health
- Norvax Compact PD vet (1-component, inactivated, oil-adjuvanted). Marketing authorisation (MA) August 2011, but on sale from 2009 under special registration exemption.
- Aquavac PD7 vet (7-component, inactivated, oil-adjuvanted). MA February 2015, on sale from July 2015.
Pharmaq
- Alphaject micro 1 PD (1-component, inactivated, oil-adjuvanted). MA November 2015, market access from April 2017 due to patent.
Elanco
- Clynav (1-component, DNA vaccine, nonadjuvanted). MA June 2017, on sale from January 2018
The project outline dated 15 May 2020 from the VI describes an arrangement where the VI has access to raw data from external stakeholders and processes these statistically. It became clear that most stakeholders are unwilling to obtain and hand over raw data for this purpose. Raw data is hard to derive meaning from compared to a report or presentation. Since adverse events in the form of spinal deformations are mostly found in harvest results, it can require a lot of resources to trace back to information on the vaccine used and the number of affected fish.
Only one aquaculture company was willing to provide raw data that included all exposures over a given period using different vaccines. From the other companies we have processed data and the stakeholders’ own conclusions. A summary of the information we received, sorted by source, is given below:
Norwegian Medicines Agency (NoMA):
Manufacturers of medicinal products are obliged to report serious events to the NoMA within 15 days of the event. Non-serious events are reported in regular reports (PV/AE cases), and for products which have been on the market for more than 5 years, such PV/AE cases are sent to the NoMA every 3 years. The Norwegian Medicines Agency summarizes the situation as follows:
“The Norwegian Medicines Agency recorded an increased incidence of adverse events for a new type of spinal deformity (“cross-stitch vertebrae” +/- connective tissue formation and melanin along the spine) in relatively large/harvest ready salmon for the vaccines Aquavac PD7 and Alpha Ject Micro 1PD especially. On this basis, the Norwegian Medicines Agency contacted the manufacturers of these two vaccines for a more detailed assessment of the incidence and causation of these adverse events. (…) the information we have received indicates that PD vaccines may be involved in these “adverse events”, but that there are most likely to be multifactorial causes. According to Pharmaq, no deformities similar to those seen in Norway have been reported for their vaccine in the UK and IE. We have received a notification of adverse events for Clynav describing deformities, but the notification does not contain enough information to indicate whether this refers to “cross-stitch vertebrae”. Clynav was introduced onto the market in January 2018 and there is currently a limit to how many Clynav-vaccinated fish are harvested.”
“The Norwegian Medicines Agency cannot draw a final conclusion on the causal relationship between spinal deformities and PD vaccines. However, we believe it is relevant that, as of today, information in the adverse events section of the summaries of product characteristics, if any, may indicate a causal relationship between the PD vaccines and “cross-stitch vertebrae”. New text for the adverse events section for Aquavac PD7 has been completed. Please refer to the attached updated SPC. The case for Alpha Ject micro 1PD is under consideration”.
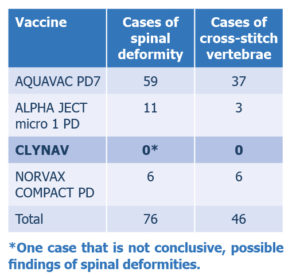
The VI has sent all the notifications from the NoMA’s records regarding PD vaccines from 01.01.2016 up to and including 13.08.2020, a total of 108 cases. The reports were received in PDF format and a lot of information is available as free text. To get an overview, we have counted the number of cases that contain information about “spinal deformations”, also called “spinal deformities” and “cross-stitch vertebrae”. There are several types of spinal deformities and the most accurate classification is achieved by means of x-ray analysis. The diagnosis of cross-stitch vertebrae is made by means of x-ray analysis and was registered for the first time at the NoMA in the course of 2017. In one of the first cases, the diagnosis was unknown and terms such as “new type of deformity” and the like were used. Some of the cases from 2018-2020 lack information on cross-stitch vertebrae and perhaps no x-ray analysis was performed. This means that the total number of cases of cross-stitch vertebrae may be higher. If one assumes that the medicinal product manufacturers have met their obligations and have the same threshold for reporting suspected adverse events to the Norwegian Medicines Agency, the number of cases for each product may give an indication of the scope. Of a total of 108 cases reported in relation to PD vaccines, 76 adverse event cases concern spinal deformities, of which 46 cases are recorded with findings of cross-stitch vertebrae (see Table 1).

Table 1. Number of adverse events per PD vaccine, reported to the Norwegian Medicines Agency relating to spinal deformities or cross-stitch vertebrae, in the period 1.1.2016-13.08.2020.
Most recorded cases were for Aquavac PD7 (MSD Animal Health) and this is also the first of the newer PD vaccines and was made available in the summer of 2015. Alphaject micro 1PD (Pharmaq) came on sale in Norway in the summer of 2017 and Clynav (Elanco Europe Ltd) came on sale in the winter of 2018. Norvax Compact PD is the oldest PD vaccine and has been on sale since 2008.
Adverse events in the form of spinal deformities are mainly recorded on salmon over 3 kg, often during quality control at the slaughterhouse. When reviewing the cases, in many cases it is challenging for the manufacturer of the medicinal product to obtain information on the proportion and number of affected individuals. In some cases, estimates are based on a reported decrease in % “superior” salmon, and information from the so-called “100-fish control”, where the reason for down-classification is registered (spinal deformations may be a category). At other times, private visits are made to the slaughterhouse with counts and the taking of sample for x-ray analysis, and in some cases it has not been possible to collect data or samples, and estimates have been based on oral information. The extent of spinal deformities varies from 70% (conservative estimate) to 1% of PD-vaccinated salmon in a slaughter pen, and the spread is greatest for cases with Aquavac PD7 (a total of 59 cases). In the 11 cases registered for Alphaject micro 1PD, there are two cases with >25% spinal deformities, while the remaining are 2-8%. For the 6 cases registered for Compact PD, there is 1 case with a maximum of 14% spinal deformities (here a low incidence of crossstitch vertebrae is indicated), in the other cases the incidence is 2.5-6%. For Compact PD, which had a monopoly in the PD vaccine market from around 2008 to 2015, no cross-stitch vertebrae were registered in 2017. Whether this is due to changes in production conditions in the last few years, or an increased awareness of spinal deformations and PD vaccination, is unknown.
The vaccine manufacturer MSD Animal Health has recently updated its Aquavac PD7 package leaflet. Increased risk of adverse events in the form of cross-stitch vertebrae, especially when vaccinating fish under 1 year old (autumnrelease), is now included. It is referred to as a “common” adverse event, i.e. occurs in more than 1 but fewer than 10 in a hundred individuals. The vaccine manufacturer Pharmaq is in process of updating its package leaflet for Alphaject micro 1 PD. The fact that both manufacturers are changing their package leaflets indicates that they accept that the products are associated with the adverse event of cross-stitch vertebrae.
NOFIMA in the person of Dr. Grete Bæverfjord:
Nofima is responsible for the ongoing FHF [Norwegian Seafood Research Fund] project 901430 “Prevention of cross-stitch vertebrae in farmed salmon”, together with INAQ, Pharmaq AS, Pharmaq Analytic and NMBU. The project started in August 2017 and will be completed in December 2020. Dr. Bæverfjord is leading the project and clearly states that there is a connection between some PD vaccines and crossstitch vertebrae. We have had access to 2 project presentations, 1 partial report from INAQ, and 2 scientific publications related to the project.
- Field data and sample materials – analysis of risk factors (responsible party: INAQ)
Production data from various salmon producers have been collected to identify risk factors for spinal deformations and the development of cross-stitch vertebrae. We have had access to a partial report, which includes salmon smolt
released in the period 2015-2017. There is no indication how many aquaculture enterprises are included or which geographical areas the data are obtained from, but since only 2 vaccine types are included (Norvax Compact PD (mono PD) and Aquavac PD7 (7-valent PD)), this indicates that the facilities are from a SAV3-endemic region where PD vaccination is common. When it comes to delimitations in method, the report says the following:
“In order to identify possible links between production factors and the development of crossstitch vertebrae, it was necessary to determine whether or not fish had developed cross-stitch vertebrae. The development of cross-stitch deformation is shown to be difficult to detect from external observation and there is no simple diagnostic test available. (…) However, fillet analysis at a slaughterhouse, where one has growth of connective tissue in the fillet, as well as malformation in the spinal column will be indicative that spinal deformation may be due to cross-stitch vertebrae. “
To determine whether fish had developed malformations of the spine, information from 3 different sources was used: 1) Quality reports from slaughterhouses with further processing (N= 122 357 fish, 105 cages, 60 autumn release, 45 spring release), 2) Fillet analyses at the slaughterhouse under the direction of the project (N= 360 fish, 20 cages, 17 autumn release and 3 spring release) and 3) X-ray analysis under the auspices of the project (N=393 fish, 28 cages, 24 autumn release and 4 spring release). All 3 data sources show a significant relationship between vaccine type and malformations of the spine. The quality reports from processing facilities were well distributed between fish released in spring and fish released in autumn, and the analyses showed the timing of release had an obvious impact:
“The predictions show that one can expect about 6% of fish to develop spinal deformation, if the fish is vaccinated with PD 7 and released into the sea in autumn. There is significant variation in the predictions associated with random impacts on the fish group, from in the region of 0 to 64% for autumn-release fish vaccinated with PD 7. There was also a negative impact from the interaction between spring-release fish and the PD 7 vaccine, suggesting that the PD 7 vaccine is associated with significantly less risk of developing spinal deformation in spring-release fish. Predictions for fish vaccinated with Norvax PD in addition to other vaccines showed a lower risk of developing spinal deformation compared to fish vaccinated with PD 7 , both for spring release and autumn-release fish” (…)
No link was found between the development of spinal deformation and the type of salmon strain, the number of non-medicinal lice treatments, or whether the feed contained glucan (used in immuno stimulating feed).
- Experimental studies – verification of risk factors and mechanisms (responsible Nofima and Pharmaq). This work is still ongoing but it was orally communicated that the incidence of cross-stitch vertebrae in this study has been low.
- By analysing vertebrae from salmon ready for harvesting, already categorised as “crossstitch” using “computed tomography” (CT), histology and scanning electron microscopy, injuries have been identified in an area of the bone tissue that may correspond to the growth zone at the time of smoltification (Holm et al 2020). It may coincide with the timing of vaccination, but primary and secondary causes of changes in growth at this stage are not discussed further in this work, and not at all in a similar article from the same project (Trangerud et al 2019)
Aquaculture enterprises
We have been in contact with a selection of aquaculture enterprises and requested access to data on the mapping of adverse events related to PD vaccination. The first priority has been the companies Lerøy Seafood, Mowi and SalMar, which in letters and meetings with the NFD expressed concern about fish welfare during the mandatory PD vaccination. None of these companies has provided access to raw data, but SalMar and Mowi have submitted processed data in the form of summaries from field studies comparing harvested or harvest-ready, PDvaccinated and non-PD-vaccinated salmon:
One company sent information from 3 facilities where they have held vaccination trials with a socalled “mark & mix” setup, i.e. the vaccine groups are labelled and mixed at the time of release into the sea and exposed to identical environmental conditions in the same cage unit. All 3 trials have been carried out fish under one year old (autumn-release fish). In facilities 1 and 2, there were two cages with a “mark & mix” setup, respectively with a 75:25 and 50:50 mixing of AquaVac PD7 (PDvaccine) and Alphaject micro 6 (non-PD vaccine). In total, findings of crossstitch vertebrae were recorded in 14.6% and 16.2% of sampled fish in the PD7-vaccinated group, compared with 6.7% and 0% in sampled fish in the Alphaject micro 6-vaccinated group. Level of severity is not indicated. In facility 3, the “mark & mix” setup comprised approx. 80:20 mixing of Alphaject micro 1PD (PD-vaccinated) vs. Alphaject micro 6 (non-PD vaccine) in two cages. Removal of 50 random fish/vaccine/ cage for x-ray analysis showed an average of 15% affected by cross-stitch vertebrae in the Alphaject micro 1PD-vaccinated group, compared to 3% in the Alphaject micro 6 group. Severe deformations were found in 10% and 2% of sampled fish in the micro 1PD group, and 0% and 2% of the micro 6 group.
In addition, we have obtained information from 1 facility (2017 generation, autumn-release), where 2 cages of Aquavac PD7-vaccinated salmon and 7 cages without PD vaccine (unnamed vaccine) have been compared. The cages with AquaVac PD7 had a lower superior proportion, and during filleting, cartilage was recorded in 46% and 50% of the PD7-vaccinated fish (0-5% in the others). Cross-stitch vertebrae were confirmed in nine out of ten affected PD7-vaccinated individuals, and the level of severity was high.
Two major aquaculture enterprises with experience of PD vaccination in the SAV3 zone have been asked to share data. We received the following statement by email from one enterprise:
“Our company (anonymised) experienced major problems with skeletal deformities and growth in 2015, 2016 and 2017 G in the sea (…) Main findings: Fish with the Aquavac PD7 vaccine had on average a risk of deformity 26% greater than fish with the other vaccine. The more days between vaccination and release, the less deformities. The higher the seawater temperature in the fi rst 60 days in the sea, the more deformities. This is consistent with the hypothesis that autumn-release fish have the highest risk of skeletal deformities when using the PD7 vaccine”.
The second enterprise sent a summary of findings after harvesting two autumn-2017 releases, vaccinated with Alphaject micro 1PD. The analyses showed that 7-8% of the fish had deformations associated with cross-stitches, but the damage was relatively limited.
Aquaculture enterprises with experience of PD vaccination in the SAV2 zone (Trondelag) have also been invited to share data/information. One enterprise has been willing to share raw data in the form of % spinal deformities during harvesting control of all production units (cages) harvested in the period 2017 to the current date (from Autumn 2015 release). This has given the Norwegian Veterinary Institute the opportunity to analyse data from a total of 202 cages. The percentage of spinal deformities was based on sampling 2×100 fish per day for quality control and categorisation of reasons for downgrading. No data are available on further classification of deformities using x-ray analysis. The model demonstrated a clearly significant association between PD vaccination and spinal deformities
(p<0.001). The dataset was unbalanced in respect of different PD vaccines, e.g. only 3 units with Clynav. Therefore, the association between diff erent types of PD vaccine and deformities was inconclusive, for example, it cannot be concluded from these data that vaccination with Clynav resulted in an increased risk of spinal deformity.
Figure 1. (Below) Proportion of fish with spinal deformities per vaccine type shown as a “Boxplot”. The line in the centre of the box is the median (the most commonly occurring % spinal deformity value), and the upper and lower wall of the box shows the area where 50% of the values are located. The 25% lowest values are located along the line under the lower wall and the 25% highest values are located along the line above the upper wall. Some of the boxes have N only equal to 2 or 3 and therefore have little information value.

When comparing PD-vaccinated fish with non-PD-vaccinated fish, the likelihood of spinal deformities when harvesting PD-vaccinated fi sh is 4.4 times greater. The model also indicated a positive significant association between spinal deformities and average weight at harvest, number of harvested fish per cage and smolt supplier, but the effect was much less than PD-vaccination.
There was no significant association between spinal deformations and timing of release (autumn or spring) as opposed to what was shown in the FHF project. One explanation might be that the FHF project mainly had harvest data from the SAV3 area, where there may be greater temperature diff erences between autumn and spring than further north. Modelling based on data from the SAV2 area predicted that approx. 4% of PD-vaccinated salmon and approx. 1% of non-PD vaccinated salmon can be expected to have spinal deformations at the time of harvesting. The FHF project did not include data from salmon without a PD vaccine, but predicted approx. 6% spinal deformations for Aquavac PD7-vaccinated salmon, with slightly greater variation than in our analyses. This shows that it is worthwhile collecting and analysing data from geographical and/or production-relevant areas where specific measures are implemented.
In addition, we have obtained summaries of analyses carried out after complaints after harvesting of 1 site vaccinated with Alphaject micro 1PD from another enterprise in the Trondelag. In this case, a prevalence of 5-8% of fish with spinal deformities and connective tissue/cartilage was assumed, and the crossstitch pathology was verified at the harvest line using x-ray analysis of three selected individuals with deformities.
Pharmaceutical companies
MSD markets the inactivated oil-adjuvanted vaccines Norvax Compact PD and Aquavac PD7.
From MSD, the VI was sent a presentation shown at the specialist conference “PD TriNations 2019”. In the presentation from MSD, a selection of field observations is shown with samples at the time of harvest. There is a large variation in the incidence of different types of spinal deformations between different fish groups, also when it comes to autumn-releases vaccinated with PD7 (in accordance with the FHF project). This indicates other unknown (production) factors that affect the risk of deformities developing, i.e. the problem is multifactorial.
Furthermore, the following is summarised from 1 controlled study, testing diff erent vaccines with and without a PD component on 1 autumn release in 2017:
- Various categories of spinal deformations are recorded for all fish, irrespective of vaccine
- Cross-stitch vertebrae were only observed in groups where the PD component was included in the vaccine
- The cross-stitch vertebrae were not visible on the X-ray analysis until 2.5 months before harvesting
- The addition of B-glucan in feed has no effect on development of cross-stitch vertebrae
Pharmaq markets the inactivated oil-adjuvanted vaccine Alphaject micro 1 PD
Pharmaq provided information that the company is in an ongoing process with NoMA of updating the package leaflet. They have informed their customers of the risk of adverse events when vaccinating autumn-release smolt.
Otherwise, please refer to information provided to the VI in November 2019 under a confidentiality agreement. This information does not address adverse events to any significant extent and is consistent with information received from the NoMA.
Otherwise, Pharmaq confirms:
For the regions south of Hustadvika, the usage pattern for PD vaccines is roughly as follows:
- Fish vaccinated with Clynav do not appear to be affected by spinal deformities and Clynav is the vaccine most used on fish less than 1 year old.
- The oil-based, inactivated vaccines are most commonly used on 1-year-olds that are not at risk of the new type of adverse events.
- Overall, approx. 98 % of all fi sh are vaccinated against PD in these areas. This is done on a voluntary basis without any instruction from the authorities. Assessment of the risk profile when using PD vaccines in the SAV 2 area must be seen in this context.
- Several of the major producers who may be going to vaccinate in the SAV2 area have successively used the same PD vaccines in the SAV3 area on a voluntary basis.
Elanco markets Clynav, monovalent DNA vaccine
Approx. 18 million doses were sold in 2018 and 56 million in 2019. Elanco provides information that, so far, they have had 1 adverse event at the Norwegian Medicines Agency, but this is not yet conclusive. The VI has received a report from NOFIMA summarising the x-ray of 477 fish from 2 groups of autumn smolt vaccinated with Aquavac
PD 7 and Clynav + AlphaJect micro 6 respectively:
22% of Aquavac PD 7-vaccinated fish had cross-stitching and 13% had severe disorder. The corresponding figure for the fish vaccinated with Clynav + AlphaJect micro 6 was 4% and 1% had severe disorder. The study cannot determine whether the impact of cross-stitching in the Clynav-vaccinated group is due to the PD vaccine or AlphaJect micro 6. This is because salmon vaccinated with standard 6-component vaccines may also have an incidence of spinal deformities with findings of cross-stitch vertebrae at the time of harvesting, but prevalence and severity appear to be low.
The Norwegian Food Safety Authority
The Food Safety Authority has received exemption applications from Måsøval, Mowi, Lerøy, AquaGen and SalMar. In addition to their own arguments, many of the exemption applications are also supported by the letter “Statement from the Aquaculture Working Group in P06, the fish-health group” sent to the NFD on 15.01.2020. Key arguments include the lack of assessment of adverse events, the lack of a cost-benefit assessment, the PD vaccines are not approved for broodstock, it is not known how existing vaccines work against SAV2, technical and adverse event problems if one also wants to vaccinate against yersiniosis, and that the vaccination instruction is late in arriving, making it difficult to change vaccination programs in time. The VI makes the following comments:
- It is true that there was little focus on the adverse events of the vaccines during the hearing process, but this was also not a major issue in any of the hearing bodies. To a greater extent, efficacy was demonstrated with the use of new vaccines and the possibility of combating PD.
- It is true that no cost-benefit assessment has been carried out.
- It is true that the current PD vaccines are not approved for broodstock, but neither are there any other vaccines with which to vaccinate broodstock in the meantime, and the vaccines are then used “off label” by the prescribing fish-health personnel making their own assessment of their use.
- Information is available on how existing vaccines work against SAV2 in the form of presentations at various technical meetings, such as PD TriNation, Dublin 2015), but the data are not published in scientific journals. There are documented immunological cross-reactions between the different genotypes (Graham et al. 2014), and the type classification for SAV is based on minor genetic differences which are not necessarily reflected in differences that affect vaccine efficacy. For example, Clynav is based on the genotype SAV2, but the efficacy of the vaccine has been documented against SAV3. It is not unreasonable to assume that Clynav works as well against SAV2 as against SAV3.
- No information is available on whether PD vaccination may affect the efficacy or adverse events of the yersiniosis vaccine (1-component). Apart from the specific “cross-stitch” adverse event, the general adverse event problems, but also the level of protection against yersiniosis, are likely to be more affected by the complex multivalent vaccine that will be administered at the same time as the yersiniosis component. However, it is possible that from a purely technical standpoint, it may be difficult to triple vaccinate against PD, yersiniosis and the remaining diseases in a single operation.
Summary of information received, the Veterinary Institute’s assessment:
- The database available to us indicates serious adverse events in the form of spinal deformities of the type “cross-stitch vertebrae” in salmon vaccinated with inactivated PD vaccines. These are Aquavac PD7 (MSD Animal
Health) and Alphaject micro 1 PD (Pharmaq) which to a greater extent are associated with this type of adverse event. Other PD vaccines in the analysis are not associated or associated to a limited extent with spinal deformities.
- However, the data material has weaknesses. In general, existing data and information from the authorities and some relevant players in the industry. The sources are the Norwegian Medicines Agency, a research project funded
by the FHF, and data collected in various ways by aquaculture enterprises and pharmaceutical producers. Only one aquaculture enterprise has granted access to production data which has facilitated its own statistical analyses.
This material can be used to indicate which variables in the dataset can be associated with spinal deformities and the strength of this association. However, we cannot say anything about causal relationships or risk factors other than those included in the submitted dataset. This indicates that the conclusions are uncertain and caution must be exercised.
- The Norwegian Medicines Agency has often made available all relevant adverse event reports from 2016 up to the present day and this information has been decisive for the VI’s conclusions in the case. In the process involving MSD Animal Health and Pharmaq, agreement has been reached on updating the package leaflets of Aquavac PD7 and Alphaject micro 1 PD. In its conclusion sent to use by email, the Norwegian Medicines Agency says: “The Norwegian Medicines Agency cannot draw a final conclusion on the causal relationship between spinal deformities and PD vaccines. However, we believe it is relevant that, as of today, information in the adverse events section in the summaries of product characteristics may indicate a causal relationship between the PD vaccines and “cross-stitch vertebrae”. New text for the adverse events section for Aquavac PD7 has been completed; please refer to the attached updated SPC. The case for AlphaJect micro 1PD is under consideration”.
- Calculations carried out in the FHF project 901430 show that it can be expected that approx. 6% of fish under 1 year old, vaccinated with Aquavac PD7, will develop spinal deformations, but that a significant variation can also be expected. Springrelease, Aquavac PD7-vaccinated smolt have a significantly lower risk.
- FHF project 901430 has not conducted similar calculations for salmon vaccinated with Alphaject micro 1 PD. Information from several different sources, as well as own analyses from the SAV2 zone, indicates that the incidence of spinal deformations in salmon vaccinated with Alphaject micro 1 PD, is probably on a par with, or somewhat lower than, that for Aquavac PD7.
- Salmon vaccinated with standard 6-component vaccines may also have an incidence of spinal deformities with findings of cross-stitch vertebrae at the time of harvesting, but prevalence and severity appear to be low.
- Our own analysis of data from the SAV2 zone shows no difference in the risk of spinal deformities at the time of harvesting between autumn-release and spring-release fish. This inconsistency with the results of the FHF project may be due to data being taken from different parts of the coast.
- Adverse events have been used as a basis for applications for exemption from the vaccination order. Extensive administration of exemptions will weaken the purpose of the order and a high vaccination level in the area.
- A high vaccination level is particularly important as the PD vaccines on the market are believed to have moderate efficacy in the field. For example, there are a large number of PD detections in the SAV3 zone each year, even though the area has a high proportion of PD-vaccinated fish. Therefore, it is important that vaccination does not replace, but supplements, other biosafety measures. There is still too little experience in the field and field data to conclusively prove the effect of Clynav.
- If Aquavac PD and/or Alphaject micro 1 PD is considered to have too high a risk of adverse events, especially for autumn-release smolt (under 1 year old), other PD vaccines are still available on the market and can be used. Norvax Compact PD has been associated with cross-stitch vertebrae, but to a much lesser extent than the above-mentioned vaccines (cf. FHF Project 901340). And apart from 1 adverse event case which is still not conclusive, no serious degree of spinal deformities has been reported in salmon vaccinated with Clynav.
- Therefore, it should be possible to adhere to the vaccination instruction without adversely affecting the welfare of the fish to an extent greater than a normal vaccination with 6-component vaccines.
(Norwegian Veterinary Institute signatories)
Edgar Brun
Dept. Director, Department of fishing health and fish welfare
Eirik Biering
Head of Section, Section for aquaculture, wild fish and welfare.
References
Holm, H., Ytteborg, E., H0st, V., Reed, A. K., Dalum, A. 5., & Bæverfjord, G. (2020). A pathomorphological description of cross-stitch vertebrae in farmed Atlantic salmon (Salmo salar L. ). Aquaculture, 735382.
Trangerud, C., Bjørgen, H., Koppang, E. 0., Grøntvedt, R. N., Skogmo, H. K., Ottesen, N., & Kvellestad,
- (2020). Vertebral column deformity with curved cross-stitch vertebrae in Norwegian seawater-farmed Atlantic salmon, Salmo salar L. Journal of Fish Diseases, 43(3), 379-389.
Graham, D. A., Rowley, H. R., & Frost, P. (2014). Cross-neutralization studies with salmonid alphavirus subtype 1-6 strains: results with sera from experimental studies and natural infections. Journal of fish diseases, 37(8), 683-691.

Clynav, Elanco and the diagonal bar logo are trademarks of Elanco or its affiliates. All other product and company names are trademarks of their respective owners. © 2021 Elanco PM-UK-20-0301

